
The main steps in the ETC include: (1) Transfer of electrons from NADH to Complex I, or from FADH2 to Complex II. The electron transport chain consists of four protein complexes (Complex I-IV) and two electron carriers (coenzyme Q and cytochrome c) embedded in the mitochondrial membrane. What are the main components and steps involved in the electron transport chain? The ETC and oxidative phosphorylation together constitute the final stages of aerobic metabolism, and are responsible for the majority of ATP generation in cells. Oxidative phosphorylation then uses the energy from this proton gradient to drive the phosphorylation of ADP to ATP by ATP synthase. This process releases energy which is used to pump protons across the mitochondrial membrane, creating a proton gradient. It plays a crucial role in aerobic metabolism, as it facilitates the transfer of electrons from the reduced molecules NADH and FADH2 to oxygen.
How does a proton gradient drive synthesis of atp series#
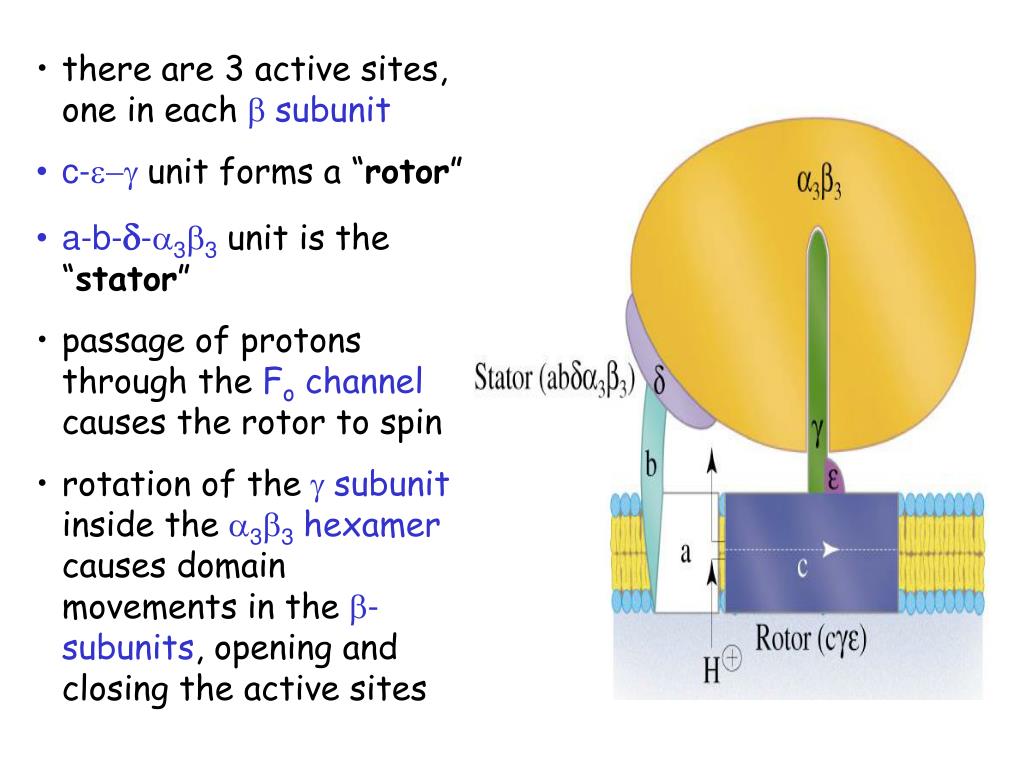
The electron transport chain (ETC) is a series of protein complexes and electron carriers located in the mitochondrial membrane. What is the electron transport chain and how does it relate to oxidative phosphorylation in aerobic metabolism? Examples: 2,4-dinitrophenol, aspirin, and thermogenin.Reduce gradient, reduce ATP synthesis, generate heat, and increase oxygen consumption.Make the inner mitochondrial membrane more permeable.Examples: rotenone (inhibits complex I), antimycin A (inhibits complex III), carbon monoxide (inhibits complex IV), cyanide (inhibits complex IV), and azide (inhibits complex IV) drugs.Block ATP synthesis by preventing electron flow across the electron transport chain.ATP synthase uses energy from protons traveling down their gradient to make ATP.Protons are transported to the intermembrane space, creating an electrochemical gradient.NADH and FADH2 electrons move along the chain.Consists of complex 1, complex 2, CoQ, complex 3, cytochrome c, complex 4, and ATP synthase.Metabolic process of making ATP using energy from electron transport.Oxidative phosphorylation & electron transport chain introduction.Examples of uncoupling agents include 2,4-dinitrophenol, aspirin, and thermogenin. This reduces the gradient, ATP synthesis, and generates heat while increasing oxygen consumption. On the other hand, uncoupling agents increase the permeability of the inner mitochondrial membrane, allowing protons to prematurely travel down their gradient. Oligomycin directly inhibits ATP synthase. Electron transport chain inhibitors block ATP synthesis by reducing the electrochemical gradient, such as rotenone, antimycin A, carbon monoxide, cyanide, and azide drugs. There are various toxic agents that can disrupt oxidative phosphorylation and ATP synthesis. Molecular oxygen serves as the final destination for electrons and is converted to water in this process. ATP synthase harnesses the energy from the gradient to make ATP in a process called chemiosmosis. NADH and FADH2 electrons move along the electron transport chain while protons are transported to the intermembrane space, creating an electrochemical gradient. This process takes place in the mitochondria and consists of several components, including complex 1, complex 2, CoQ, complex 3, cytochrome c, complex 4, and ATP synthase. Oxidative phosphorylation is the metabolic process of making ATP using energy obtained from the transfer of electrons in the electron transport chain.


 0 kommentar(er)
0 kommentar(er)
